What is cGMP for the 21st Century?
As many reading this will know, cGMP, stands for current Good Manufacturing Practice. It is the quality management system (QMS) FDA mandates for companies developing, manufacturing, and distributing medicines, and other pharmaceutical products, in the United States. The applicable regulations are contained in the Code of Federal Regulations, Title 21.
So, with the 21st Century having been with us for 25 years now, why am I writing about the prospect of cGMP entering the 21st Century? Isn’t it already here?
That’s what we are about to explore, recognizing that we will be comparing the performance of pharmaceutical companies, and their supply chains, with today’s highest performing industrial sectors.
We begin with a journey back in time, to establish progress, or otherwise, during the 20th century.
The Pharmaceutical Company in 1979
The question takes me back to my early days in the industry, when I joined Miles Laboratories, a pharmaceutical manufacturing and packaging plant, based in Bridgend, South Wales. Soon after, it was acquired by Bayer AG.
When I joined Miles, cGMP was very much more contained than it is today. The industry was dominated by large, vertically integrated companies that carried out the research, development, manufacture, and distribution of their own products. Figure 1 shows a diagrammatic representation.
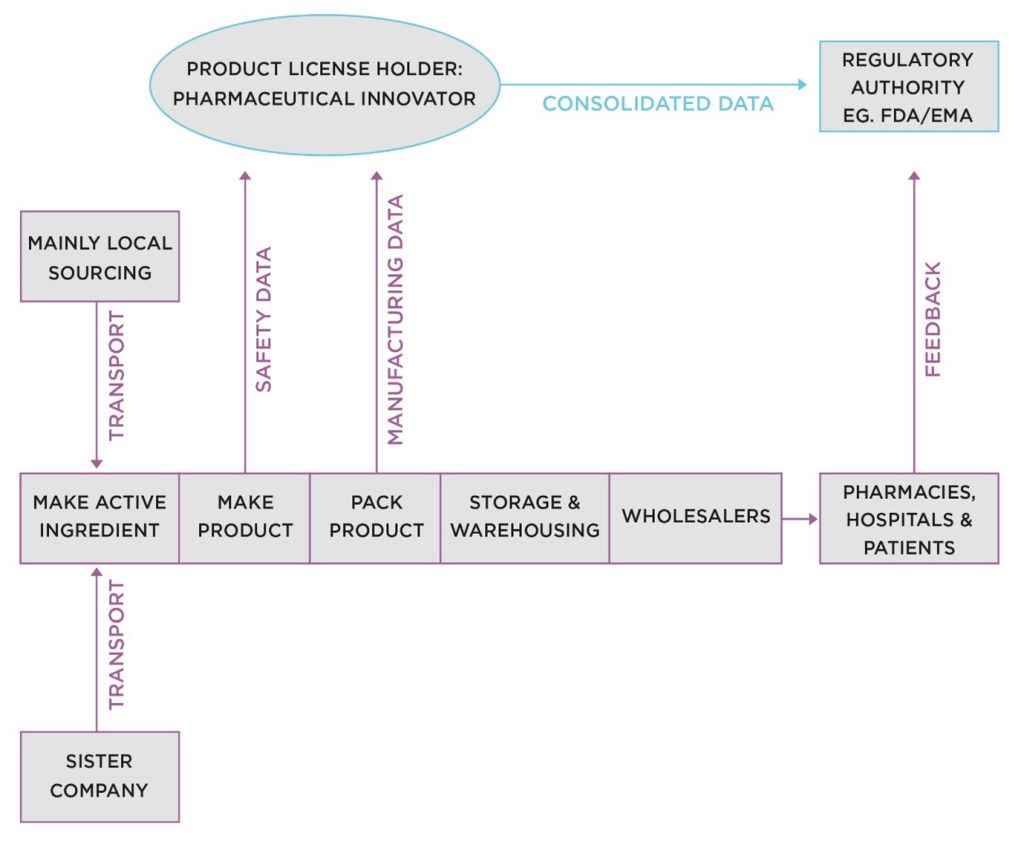
Figure 1: Diagram of the Industry Structure in 1979.
In Figure 1, we see a single company (acting as clinical trial sponsor and product license holder) carrying out the entire development program, submitting a filing to a country regulatory authority for review and a potential approval to sell.
The site of manufacture covered all conversion activities, from receipt of raw and starting materials at goods inwards, through the various manufacturing processes, to the dispatch of finished, packaged products to wholesalers—often directly to hospital and community pharmacies.
The site QMS, was under single ownership of the site. There was no mistaking who was responsible for compliance and Corrective and Preventative Action (CAPA) – the product license holder (PLH)!
The Site Master File was regularly updated and on hand for the inspections. It clearly outlined the company’s policies and procedures with respect to site operations and quality.
Regulatory inspections and audits would start with a review of the Site Master File, followed by a physical plant inspection and review of all relevant documentation, such as standard operating procedures (SOPs) and batch manufacturing records. The entire development, manufacture, and shipment of each product was carried out under the one ownership, one roof.
Shipments to countries ex-UK went direct from the plant, into Bayer owned warehouses for in-country distribution.
With this level of integration, customer complaints could be dealt with effectively. It was not unusual to receive the polystyrene packing piece from the top of a glass bottle (who remembers those?!) of Alka Seltzer from a customer, stating it would not dissolve! We were able to resolve the confusion in no time, often with a note of apology, a complementary sample, and a wry smile from the staff.
Product recalls were exceptionally rare and would place little strain on plant capability to carry out forward and reverse traceability—all movements were under the one roof, except for raw and starting material sourcing.
During the intervening years, there was an explosion of business models and supply chain actors in the industry, as shown in Figure 2.
Figure 2: Industry structure as it is today.
This picture in Figure 2 is very different from the 1979 version in Figure 1. The supply chain at the bottom has a large component of contract manufacture added to it, carried out by contract development and manufacturing organizations (CDMOs). We also see the move towards global, offshore sourcing, mainly focused on Asian countries.
These transitions firmly established themselves from the mid-1980s onwards, remaining the incumbent modus operandi today. In order to support the changed strategy, the need for third-party logistics services grew accordingly, as supply sources and CDMO locations continued to globalize.
along with offshoring procurement policies. Transportation runs through every stage of manufacture, for both in-house and contractor owned establishments.
We see there are also a lot more interconnections involved in developing drugs, as the R&D-based innovator companies increasingly outsourced early-stage development to SMEs. Along with that, biologic products have gained favor, adding additional complexities, such as sensitivity to temperature variation, yield and potency uncertainty, and lack of interchangeability due to different processing methods.
For Regulatory Authorities, the picture has changed dramatically. In 1979, they dealt with a limited number of large, experienced pharmaceutical companies, with a known track record. Mutual trust, based on the omnipotence of patient safety, was the working principle.
As it stands today, the number of interactions with companies and business models developing drugs has grown exponentially, leading, understandable, to shallower relationships and less sharing of knowledge and understanding.
Finally, and possibly the most concerning element of the supply chain evolution, is the relationship between pharmaceutical companies and the distribution channel.
Figure 3 shows how it is today.
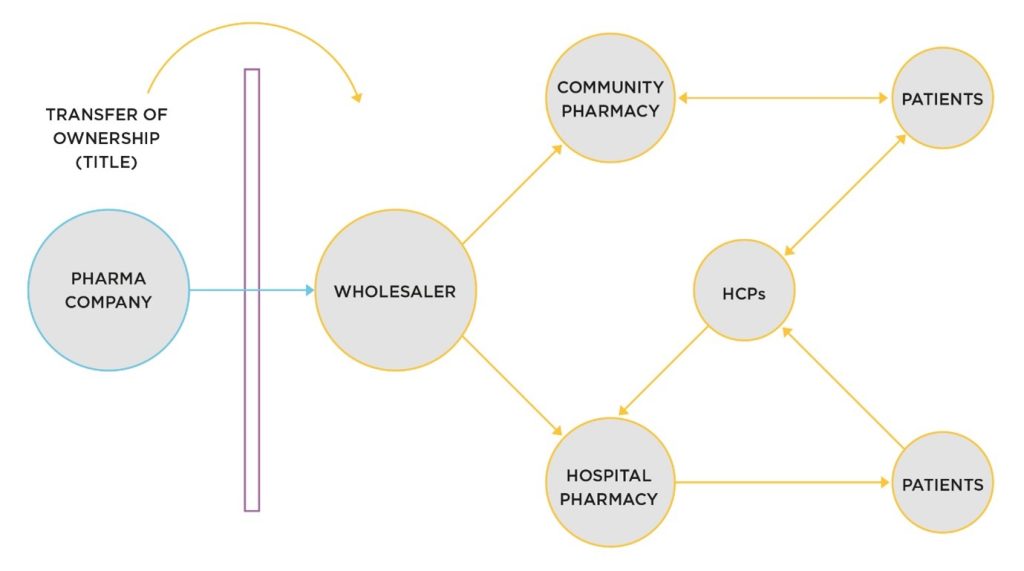
Figure 3: Transfer of ownership to the distribution channel
We see that wholesalers purchase finished goods from pharmaceutical companies with licenses to sell. Once under the ownership of the wholesaler, the pharmaceutical company cannot be, and is not, involved. It is a one-way flow of goods.
The consequences is that the holder of the product license is unable to fulfil many of its cGMP obligations to the quality of their product in circulation by and the associated pharmacovigilance activities.
Is cGMP for the 21st Century a pipe dream then? Or is there room for optimism
From the above, there seems little room for optimism in the pharmaceutical supply chain transitioning into the 21st Century.
In Part 2, we will assess the scale of the challenge ahead, if meaningful progress is to be made.